Introduction
For years hair transplant physicians have contemplated holding solutions for grafts, however, very few studies comparing holding solutions in hair transplant surgery exist. There are many studies looking at individual holding solutions. Some studies indicate that inexpensive solutions such as normal saline or Ringer’s Lactate are sufficient while others suggest that more expensive solutions such as Hypothermosol, Viaspan, Wisconsin Solution, or Platelet Rich Plasma are superior. There is also the question regarding temperature. Should we chill our grafts and, if we do chill them, what is the ideal temperature? What happens when we chill tissue in the specific storage solutions? Could chilling grafts in some solutions actually be dangerous to our grafts? More recently, some have considered the addition of antioxidants, ATP, and oxygen to their holding solutions. In fact, there is a new product under research called Vitasol, which has liposomes containing ATP. What are the benefits of such a product if any?
Most work done on organ transplants has looked at larger organs such as the liver, kidney, liver, pancreas, and myocardium. There has been comparatively little basic science studying the survival of hair follicle grafts.
Studies
The following is a review of the literature with the hope that it might stimulate further study in this important topic. There have been a few studies looking at storage mediums and temperature but they have failed to show any reproducible evidence that any specific protocol improves the survival of hair. In addition, the design of many of these studies do not withstand statistical scrutiny. A study by Kim found that storage at 4°C in normal saline does not improve graft survival unless the grafts are kept out of the body for more than the first 6 hours. After 6 hours preserving grafts at 4°C appears beneficial, but after 6 hours follicular survival decreased whether or not the follicles were preserved at 4°C.1 Perez-Meza found no difference in the survival of grafts at ambient temperature vs. 4°C between 4-6 hours.26 A study by Beehner found that cold storage in normal saline improves the survival rate of grafts exposed to crush injury.2 Uebel observed that the survival of hair increased by 15% when stored in platelet rich plasma (PRP), though this has not been reproduced or confirmed.3 Cooley has suggested that free radical scavengers may protect against reperfusion injury, but no statistically significant studies have shown that they improve hair graft survival.4 He also noted that oxygen might benefit the survival of hair follicle grafts, and later suggested we add ATP.5 Following a study using mice, Qian proposed that a 0°C storage solution is superior to the more commonly used 4°C for the long-term preservation of hair grafts.6 Raposio found no statistically significant difference between the survival and growth rates of grafts stored at room temperature and those preserved at 1°C.7 Krugluger demonstrated that transient effluvium was prevented in 6 patients whose grafts were stored in PBS buffered tissue culture medium (TCM) with nitric oxide added as an inhibitor of apoptosis.8 In another study, Krugluger found that grafts stored in PBS containing B12 revealed increased elongation of the hair follicle grown in tissue culture compared with those stored in PBS alone.9 However, it remains unclear whether elongation is truly a good indicator of graft vitality. Parsley presented a photograph of a single patient in which he compared graft growth following storage in Hypothermosol on one side and plasma-lyte A on the other side. The graft growth appeared more robust on the Hypothermosol side.27 Beehner found that grafts stored in chilled Hypothermosol and ATP improved the survival rate of grafts over chilled normal saline at a variety of storage times ranging from 2 hours up to 96 hours with the exception of the 6-hour mark.10 The author (Cole) showed that the pH declined in normal saline regardless of temperature as a function of time, but pH remained constant in PBS, which contains buffers.11
Beehner Study
The Beehner study comparing chilled saline survival rates to those survival rates of Hypothermosol containing ATP concluded that grafts stored in chilled Hypothermosol with ATP have a better yield than grafts stored in chilled saline over the same period of time.10 The survival rates are noted in Table 1.
Beehner study comparison of hair follicle yields from chilled saline vs. chilled Hypothermosol with ATP.
This is the first study documenting that a chilled intracellular (Hypothermosol) storage solution is superior to a chilled extracellular (normal saline) storage solution with hair grafts. (The terms “intra-” and “extracellular” are very important and are discussed further below.) In this study, Beehner planted 10 single-hair grafts and 20 two-hair grafts into boxes at 2, 4, 6, 8, 24, 48, 72, and 96 hours after strip harvesting. This study also demonstrated that the quality of the hair in terms of follicular diameter was superior with grafts stored in Hypothermosol and ATP with the exception of follicles planted at the 6-hour mark.
A previous study by Limmer examined the effect of chilled saline solution on the survival rate of grafts stored up to 48 hours prior to transplantation.12 Leaving aside the issue of whether further statistical analysis is necessary in both studies in view of, for example, the 4-hour survival being no better than 48-hour survival, the follicle survival rate in Beehner’s chilled saline study was far less than in Limmer’s study with the exception of 6 and 8 hours out of body. The comparison suggests that chilled saline may produce a lower survival rate (Table 2).
Comparison of Limmer’s and Beehner’s studies on hair follicle yields from chilled saline.
Specific solutions seem to offer advantages to the individual organ transplanted.13 While a variety of standard solutions are used to preserve tissue, standard solutions with individualized additives (custom solutions) designed to meet individual organ biological requirements improve the viability for different tissue types.14 With regard to hair, no optimal protocol, solution, or temperature has been defined. With this in mind, we will look at the basic science behind biopreservation.
Basic tenants and theories. The environment of the extracellular space is vastly different from the intracellular space. Cells maintain a stable environment through the energy/ATP consuming sodium and calcium pumps that control cell volume and ion distribution. In addition to inorganic ions, cells contain a variety of organic molecules and macromolecules that are not free to cross the bi-layer phospholipid cell membrane. The extracellular osmolality is controlled at about 300m osmol/kg. Cells will tolerate a perturbation of osmolality of only 10% without harm.15 If the sodium pump is inhibited through energy deprivation or hypothermia, the impermeant solutes and colloidal material inside the cell result in water uptake and swelling of the cell. As water dilutes the concentration of salt inside the cell, more salt enters the cells, which leads to more water influx. Eventually, the cell membrane ruptures or bursts.
Cells derive energy (ATP) from the oxidation of glucose through glycolysis, the citric acid cycle (Krebs cycle), and the electron transport chain. The glycolytic pathway oxidizes glucose into ATP and pyruvate. In aerobic conditions, pyruvate is oxidized to form ATP through the citric acid cycle and the electron transport chain, while in anaerobic conditions pyruvate is converted to lactic acid. The only source of ATP in anaerobic conditions is through glycolysis (2 ATPs vs. 37 via aerobic conditions).
The oxygen requirement for many tissues is quite high, but the solubility of oxygen in tissue is quite low. Interruption in circulation rapidly leads to inhibition of aerobic energy production. Loss of circulation also deprives cells of necessary metabolites and eliminates removal of waste products.
Excision of grafts for transplantation results in ischemia. The decisive event is ATP depletion, which occurs within 1-2 minutes of oxygen deprivation.16 This early event leads immediately to a shift from aerobic to anaerobic metabolism, which very quickly becomes self-limiting with the production of lactate and H+ (i.e., lactate and H+ have a negative feedback effect on ongoing glycolysis, the anaerobic means of ATP production). Other events also occur early including cellular depolarization, a rise in intracellular protons (acidosis), and an increased intracellular calcium. Mitochondrial oxidative phosphorylation is the first casualty of ischemia. Such events contribute to cell death. Ischemia in hair transplant surgery can last many hours and often exceeds 4-6 hours. Advances in holding solutions and temperature reduction may offer ways to improve hair transplant survival.
For each 10°C decrease in temperature, oxygen consumption decreases by approximately 50% and is called the Q10. The oxygen consumption at 5°C is about 6% of that occurring at 37°C. Cooling provides short-term in vitro survival primarily through the decrease in metabolism and the reduction in oxygen and nutrient demand and a consequent conservation of chemical energy. Hypothermia is far more than the single variable of reduced temperature, however. Hypothermia will protect only to a point. Hypothermia without manipulative intervention may lead to progressive cell injury during each of its three in vitro phases (cooling, maintenance in the cold, and re-warming). Fluctuation in temperature (chilling, warming, and re-chilling) may result in even greater stress to the cells. Perhaps it is best to keep the cells at a specific temperature rather than a fluctuating temperature whenever possible, although, if this were at warmer temperatures, the resultant increased energy consumption would possibly present a new set of problems. The rapid change in temperature of the hair follicles from 8°C to 37°C can induce apoptosis, and at the very least is a “shock” to the cells of the hair follicle. Gradual warming may be a more optimal protocol for cell survival.18
With hypothermia, the biopreservative solution is reduced from 37°C to 20°-25°C or, more commonly, 0-10°C. The target of 4°C has no rationale other than that water reaches its maximum density at this temperature. Nevertheless, the 4°C temperature is the one most often quoted by hair transplant surgeons with regard to chilling their grafts. Cooling, however, has its own characteristics that result in a depletion of energy stores and the associated adenylates (esters of AMP) due to the failure of aerobic production of ATP and a subsequent failure of the energy-dependent ion pumps in the cell membrane with subsequent rapid gains in calcium, loss of intracellular K+, and a gain of Na+ and Cl– as well as an increase of osmolarity and associated cellular swelling. The pH also falls with an acidosis approaching a pH of 4 since without oxygen the pyruvate from glycolysis becomes lactic acid rather than being further oxidized. There is also a change in cell and organelle membranes, which become “leaky.” In addition to the production of free radicals and depleted natural defense mechanisms to the free radicals, cooling provides multiple pathways for the initiation of apoptosis.17 The lipid bilayer membrane may change phase to a gel state at a lower temperature from a more fluid state at a higher temperature. As you can see, cooling by itself can be harmful to cells.
Different cell types have different tolerances for maintaining optimal health.19 For example, the ischemic tolerance of the brain is only 6 minutes at 37 degrees, but is extended to nearly 60 minutes when the body temperature is reduced to 17 degrees.16 Kidneys are able to tolerate much longer periods of “warm ischemia” (i.e., nonhypothermic ischemia). Total necrosis of the majority of tubules occurs after 60 minutes. As indicated by Kim, hair follicles at room temperature tolerate even longer periods of ischemia.1
Therefore, it makes sense to look for a preservation solution and temperature that is ideal for the hair follicle. It is important to approach storage solutions with a molecular-based logic to design the ideal holding solution for the specific cell types that comprise the hair follicle. Solutions should support the cellular proteome, genome, and fragmentome in addition to cellular structures such as the mitochondria, cell membrane, and nucleus. The proteome is all the proteins expressed by a genome and the fragmentome refers to the peptide fragments.
Cost of various products extant today:
As you can see, the cost varies greatly between the different storage solutions, so it is important to examine the differences in the various storage solutions.
Storage Solutions
Intracellular storage solutions. These are hypertonic solutions with elevated K+ levels and reduced Na levels more similar to the intracellular space along with a larger anion that is unable to cross the cell membrane and will therefore provide osmotic support. This latter quality is important because with reduction in temperature, water-controlling cell pumps that regulate the osmotic gradient are inactivated. The higher osmotic pressure found in these storage solutions inhibit passive influx of water and thereby reduce the risk of cell swelling. Examples of intracellular storage solutions include Hypothermosol, Collins, Euro Collins, Viaspan (University of Wisconsin Solution), CryoStor, Celsior, HTK-Custodial, Unisol, and KPS1.
Tissue stored in intracellular storage solutions should be chilled, but at what temperature? According to Dr. Abey Matthew, grafts stored in Hypothermosol should be kept below 12°C and above freezing with a recognized usage range of 2°-8°C.20 The composition of Hypothermosol is noted below:19
Extracellular storage solutions. This is an isotonic solution having a plasma-like complement of ions that mimics the normal extracellular environment of cells. Examples include normal saline, Ringer’s Lactate, BSS, and tissue culture media that mimic the extracellular composition of plasma and other body fluids. Such solutions are poor preservation solutions at reduced temperatures. These solutions can lead to cell swelling at lower temperatures due to reasons explained above. For this theoretical reason, extracellular storage solutions should never be chilled. Nevertheless, these solutions are the most commonly used and are used in combination with hypothermic temperatures.
To summarize, there are strong hypothetical reasons based upon decades of large organ transplant research that conclude the following:
Synthetic preservation solutions designed specifically to inhibit detrimental cellular changes that ensue from ischemia and hypoxia may be better than culture medium or saline, which are commonly used clinically.
Chilling of grafts should only be done when the grafts are stored in an intracellular medium.
If the physician is using an extracellular medium to store grafts, the tissue should not be chilled.
However, not chilling the tissue presents its own problems as the metabolic rate and associated ATP demands are not decreased while the supply of ATP is dramatically diminished with the loss of normal perfusion of the tissue.
The minimum essential characteristics for the ideal storage solutions address the following:
Minimize cell and tissue swelling
Maintain appropriate ionic balance
Prevent a state of acidosis
Remove or prevent formation of free radicals
Provide substrates for the regeneration of high energy compounds (e.g., ATP) and stimulate recovery upon re-warming and reperfusion
Beyond these basic minimal components, new cryoprotective strategies are emerging that primarily focus on combating oxidative stress and cold or hypoxia-induced apoptosis. Lactobinate found in Viaspan, Hypothermosol, Celsior, Cardiosol, Churchill’s solution, and others is a strong chelator of calcium and iron and may, therefore, contribute to minimizing cell injury due to calcium influx and free radical formation. Calcium is a major factor in apoptosis. Other anions included in an ideal solution include gluconate, citrate, glycerophosphate, and anionic forms of aminosulphonic acids such as HEPES. Osmotic agents such as sucrose and mannitol are often included. Mannitol possesses properties such as hydroxyl radical scavenger and prostaglandin-mediated vasodilatation. Macromolecular oncotic agents include human serum albumin, hetastarch, or hydroxyethyl starch (HES). Dextran-40 is a colloid for oncotic support that can improve the efficiency of the removal of erythrocytes by inhibiting red cell clumping. High magnesium concentrations and very low calcium levels have shown value in cardioplegia and myocardial preservation. Some glucose is often included as a substrate but at a low concentration to prevent exogenous overload during hypothermia, which may potentiate lactate production and intracellular acidosis by anaerobic glycolysis. HEPES is an excellent buffer at low temperatures. Adenosine is an essential substrate for the regeneration of ATP during re-warming and also acts as a vasoactive component through vasodilatation. For this reason, some suggest the inclusion of ATP in graft storage solutions. Glutathione is an important cellular antioxidant and hydroxyl radical scavenger, as well as a cofactor for glutathione peroxidase, which enables metabolism of lipid peroxides and hydrogen peroxides, both potent free radicals. Finally, one might include molecules for oxygen delivery, calcium channel blockers, apoptosis inhibitors, and trophic factors.
Vitasol, liposomal ATP, contains 2.5mg/ml lipid with 5mM ATP. Cells and tissues (of the hair follicle size range) are best kept for extended periods of time at concentrations of 0.01-1mg/ml lipid and thus ATP concentrations of 0.1-2mM ATP to minimize purinergic effects. The purinergic effects will still occur, but at 0.1mM-1mM concentration range the P2Y receptors are activated and actually inhibit apoptosis. For this reason, Vitasol should be diluted 1:10 in normal saline or Hypothermosol.18 Transitioning cells from a chilled state to room temperature in normal saline with Vitasol prior to warming to body temperature reduces “shock” to the cells and may have other benefits. At room temperature cell metabolism is still reduced, which decreases ATP consumption. In addition, tissue stored at 14°-20°C is optimal for the uptake of the ATP by cells.18
Reperfusion Injury
It is well known that hypoxia does not always cause tissue damage, but reperfusion of tissue shows marked and occasionally severe tissue damage. The reperfusion stage is when the ischemic organ is re-implanted and exposed to the body’s circulation. There are several hypotheses as to the cause of this injury. These include free radicals, inflammatory cells, intracellular calcium accumulation, and loss of membrane phospholipids. Reperfusion injury is one theory behind poor growth that is occasionally seen in hair transplant surgery.
A free radical is a molecule with an unpaired electron denoted by a dot (R*). The electron renders the molecule highly unstable and reactive. The high reactivity initiates chain reactions that produce toxic free radicals.21 (Under normal conditions cells produce small quantities of free radicals since the energy of the electron is transferred to intermediate molecules such as NAD, FAD and ADP with the energy/electron/photon ultimately being stored as ATP.)
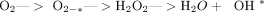
The hydroxyl free radical is the most reactive of all free radicals and will oxidize any organic molecule almost instantaneously. Superoxide dismutase detoxifies most of the free oxygen radicals made during a cell’s normal metabolism. With reperfusion injury, the normal protective enzymes may be overwhelmed by the electron energy that couldn’t be stored as ATP due to, among other things, the absence of O2. In addition, in ischemia, ADP becomes hypoxanthine, which is further converted to xanthine. This loss of substrate for ATP formation would appear to be a major impediment for the resumption of normal cellular respiration. Furthermore, during reperfusion, xanthine is converted into uric acid and superoxide anion (2O2-*). Other endogenous antioxidants such as glutathione, glutathione peroxidase, catalase, ascorbate (vitamin C), alpha-tocopherol (from vitamin E), and NADH act as free radical scavengers, but may be depleted during reperfusion.22 Other drugs that may help include Allopurinol and compounds that chelate iron. Poor growth from hair transplant surgery may be minimized by use of free radical scavengers in graft holding solutions.
Optimal Temperature
Every organ has an optimal temperature for storage based on the interaction of hypothermia, the nature of the cell, and its chemical composition. For cardiac muscle, the optimal temperature is 10°-20° Celsius.23 For the kidney, some studies show that 10° Celsius is superior to 5° and 5° is superior to 0.5°.23,24 We are yet to study the optimal temperature for hair grafts. Parsley, for example, feels the optimal storage temperature for hair follicle grafts is between 8°-14° Celsius, but he bases this on optimal temperatures for other organs rather than for hair follicles.26
Chilling Stations
I noted for years that the ice seemed to melt after a few hours and the temperature rose abruptly in my storage medium. To overcome this shortfall, I began to fill my PVC containers with water and freeze them in a larger freezer. Upon taking them out of the freezer, I insulated them and placed an aluminum plate over the ice because it transferred heat from my grafts more effectively. The added insulation, the aluminum plate, and the full container of ice helped to keep the temperature below 10°C for over 6 hours.
Unfortunately, personal experience shows that the temperature may fall well below 4°C abruptly and stay at this temperature for a prolonged period of time in an ice-based system. We do not know if the temperature below 4°C has a negative impact on the survival of hair follicles. Furthermore, we do not know if the abrupt drop in temperature is as good as a gradual decline in temperature. Some theorize that a gradual decline in temperature is better for hair follicle grafts. Dr. Parsley first moves his hair follicle tissue to a room temperature holding reservoir and then transfers it to an ice-based system to achieve a more gradual decline in temperature from body temperature.25
We know that tissue is traumatized when it is taken from the body. It is further traumatized when it is chilled. Finally, it is traumatized once again when it is warmed. Based on this theory of traumatization, it may be harmful to our grafts if we allow our grafts to rise above the low temperature in our holding solutions to a temperature closer to room temperature and then suddenly shock them again by chilling them. Perhaps we should strive to maintain a constant temperature throughout the chilling process based on this rationalization.
Consequently, I have created a chilling pump that circulates a cool mixture of propylene glycol through my storage dishes. I’ve found that this system allows me to maintain a constant temperature and may benefit someone interested in doing studies into the optimal temperature for graft storage. More recently, I created the Graft Chilling Plate (GCP). The GCP is a compact chiller designed to hold one or two petri dishes and is capable of storing grafts at a stable temperature throughout the duration of the hair transplant procedure. It will hold multiple stainless steel cones (developed by Dr. Bill Parsley) that allow the transfer of multiple grafts at one time.
Discussion
There is no ideal temperature or holding solution for hair restoration surgery based on current studies. One must define what the optimal outcome would be from a holding solution. We should also attempt to uncover the optimal storage solution and temperature for hair follicle grafts.
Some theorize that the ideal holding solution will prevent anagen effluvium. Dr. Krugluger created the Moser solution, which he reported prevented anagen effluvium.8 Unfortunately, this solution has never made it to the market and there is no long-term data to support his conclusions. Furthermore, transfer of the hair in grafts obtained by FUE to the scalp in less than 20 seconds has not been shown to prevent anagen effluvium either.28 One would question whether a holding solution would be of benefit in preventing anagen effluvium if such rapid transfer failed to prevent it. Furthermore, the prevention of anagen effluvium would be the result of the prevention of apoptosis of the more metabolically active progeny of the stem cells, whereas the more important cell to protect may likely be the much less metabolically active stem cell, especially the mesodermal stem cell. This would seem a lower bar to hurdle and would make hair mass per hair follicle a more relevant outcome parameter.
The most important benefit of holding solutions would be an increase in hair yield from the transplanted grafts. The optimal holding solution would reduce the trauma to tissue from reperfusion injury and free radical formation as well as from ion and osmolarity abnormalities created by the ischemic phase. As Beehner has shown, chilled grafts seem to tolerate crushing trauma better than grafts maintained at room temperature.2 His follow-up study that compared room temperature normal saline to Hypothermosol and ATP concluded that chilling tissue in Hypothermosol and ATP resulted in a higher yield of hair. Another potential benefit from the optimal holding solution and the optimal temperature include the potential to prevent the growth of thinner hair shafts, which will probably be found to represent a spectrum whose extreme is no growth. There might even have been found a way to “supercharge” the hair fiber diameter as Cole noted happening naturally in the donor area adjacent to the strip scar.29
We know that survival of many organs improves with cooling. We also know that certain tissues respond better to specific temperatures. Furthermore, some tissues respond better to a specific or customized holding solution. It remains for us to define these in terms of hair follicles. We remain uncertain whether there is any benefit from chilling grafts below room temperature. We do know, however, that, for reasons discussed, there is strong evidence with the transplant experience with other organs that suggests that only hypertonic solutions should be used when chilling grafts. Furthermore, that data also suggest that isotonic solutions should be used when grafts are maintained at room temperature. Despite this knowledge, hair transplant data at this time suggest that chilled grafts in normal saline or Ringer’s Lactate seem to thrive just as well as when they are placed at room temperature in these solutions. It is uncertain whether follicle survival is better in hypertonic solutions at a cool temperature as is the case with other organs. There are a variety of different holding solutions of both the intracellular and extracellular composition. For reasons stated in this article, extracellular holding solutions should not be chilled. Conversely, as discussed, grafts should not be stored at room temperature in intracellular holding solutions.
Despite the research to date, there is no conclusive evidence to guide us. Perhaps with time the unknown variables will become clarified. Fortunately, whatever we do with regard to holding solutions seems to produce “acceptable results.” This speaks to the heartiness of the hair follicle. It seems to survive trauma, including iatrogenic trauma, quite well. Acceptable results, however, should not mollify us into complacency.
- Copyright © 2012 by The International Society of Hair Restoration Surgery