Optimal graft growth is mainly dependent on surgical technique. This includes harvesting and creating grafts without transection, avoiding dehydration, and implanting grafts into the recipient sites without trauma. But other factors are likely to contribute to the results as well. This article will discuss these contributing factors and the treatments that have been developed to address them. If surgical technique is the “cake,” then these bio-enhancements can be thought of as the “icing on the cake.”
But first, a word about “evidence.” Clinical researchers agree that proper studies must conform to certain rules to be considered legitimate. For example, there must be enough subjects so that any differences between the treatment and control are not due to chance. When it comes to hair transplant outcomes, there are essentially no studies that meet these minimum standards, because they are virtually impossible to perform. These randomized, controlled trials are the highest form of evidence, but there are other forms of evidence as well. Clinical observations, case reports, and expert opinions constitute lower forms of evidence, and it is primarily this type of evidence that has propelled our field forward over the past two decades. This is the type of evidence that supports bio-enhanced hair restoration.
I would like to share my clinical observations and opinions about bio-enhanced hair restoration. I define “bio-enhanced hair restoration” as the utilization of biologic-based products and techniques in the medical and surgical treatment of hair loss. These include growth factors, extracellular matrix products, platelet rich plasma (PRP), tissue holding solutions, adenosine triphosphate (ATP), and other naturally occurring substances (Figure 1). Usually, these have been developed for other fields, such as wound healing and regenerative medicine.
Liposomal ATP
Many physicians agree that physical trauma to the graft during the procedure is the biggest factor in reducing graft survival. Which factor would be the next most important? In my opinion, it is blood flow, or oxygen supply, to the grafts. When a hair follicle is transplanted, the graft must wait about 5 days to be reconnected to its own dedicated blood supply. What is amazing to me is that grafts ever grow at all! Evidently the amount of oxygen flowing through the scalp is enough to diffuse into the cells of the graft most of the time. If the oxygen is not enough (ischemia), there may be either loss of the entire follicle, or just a percentage of the cells in the follicle, resulting in new hairs that are finer and weaker.
Several years ago, I measured scalp oxygen levels in my patients undergoing hair transplantation using a device that measures visible light spectroscopy (Spectros T-Stat). I found the results rather surprising. Compared to readings in the fingertip (which were uniformly high) and the ankle (which were uniformly low), oxygen readings in the scalp varied greatly from one patient to the next. Furthermore, when a vasodilator was applied to the scalp, oxygen levels increased but the degree of change was again highly variable.1 This suggests that both baseline scalp oxygen levels and the amount of vascular “reserve” vary greatly from patient to patient. This may be one explanation for the variation in graft survival we see in our patients.
If patients have such a wide range of blood flow and oxygenation, what can be done to address this? Certainly the recipient sites can be made in such a way as to minimize damage to the vascular bed. As we increase the density of our sites, we increase potential injury to the vascular bed; furthermore, by placing more oxygen-starved grafts per cm2, we are increasing demand. This problem of “increasing demand-decreasing supply” explains why many have observed occasional growth problems at higher grafting densities.
When I did my scalp oxygen studies, I also looked at ways of increasing skin oxygen levels, including hyperbaric oxygen. While the possible benefits were there, the practicality was not. For a period of time, I even tried topical oxygen with encouraging results,2 but again practicality limited its usefulness. At the time I was doing this research, I found several references to a vasodilator containing nicotinate being able to raise skin oxygen;3 after experimenting with it, I came to the opinion that there was some possible benefit there. Unfortunately, individual sensitivities to topical vasodilators vary, some patients have no response and other patients flush and get light-headed!
In 2005, Dr. Bill Parsley introduced me to Bill Ehringer, who was at that time a physiology professor at the University of Louisville. Ehringer had developed and patented a liposomal version of adenosine triphosphate (ATP). After much trial and error, Ehringer’s team had created a very specific type of liposome that was able to fuse with the cell membrane and deliver the ATP inside the cell.4 They were interested in what the liposomal ATP would do as an additive to graft holding solutions. I was much more interested in what it would do as a post-operative treatment for the grafts. I reasoned that if it took up to 5 days for grafts to become revascularized, adding ATP during this time may be beneficial to make up for any shortfall in oxygen.
Over the ensuing years, I tried different strengths and formulations of the liposomal ATP as a post-operative spray; I gradually settled into a protocol that worked well for me. Healing seemed to be enhanced, but more importantly, my graft growth “variability curve” seemed to be shifting to the right (i.e., fewer cases of poor growth, better average results, and more “wow” results). I don’t think variability can ever be entirely eliminated from hair transplant results because of all the possible factors that can affect growth, but having the patient spray liposomal ATP on their scalp appears to have a significant positive impact. Several colleagues who have adopted our protocol using liposomal ATP have reported the same thing. Reports in the peer reviewed medical literature prove that liposomal ATP has the ability to protect ischemic cells,5-7 so it is reasonable to suggest that it will benefit ischemic hair follicles.
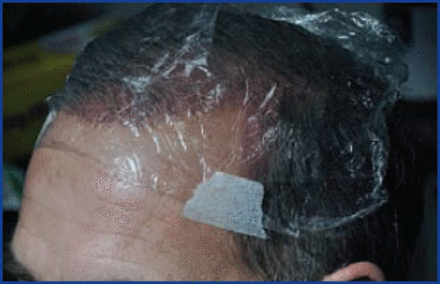
How we use ATP: the liposomal ATP (available from Energy Delivery Solutions) comes as a concentrated solution that needs to be diluted for clinical usage. As a holding solution additive, we add 1cc concentrated ATP to 100cc of HypoThermosol FRS. For the post-op spray, we add 10cc ATP to 90cc of saline in a spray bottle we give to the patient. We have them spray every 1-2 hours for the first 48 hours (including waking up the first two nights), and then every 3-4 hours thereafter while awake. For the first couple of days, the patients keep their scalp covered with kitchen cellophane to keep the moisture in, similar to a greenhouse (Figure 2).
Holding Solutions
Going back to Ehringer and Parsley’s original interest in ATP as an additive to holding solutions, it seemed to make sense that exogenous ATP would help cells keep functioning while being stored out of body. As a review, graft holding solutions potentially protect grafts from “storage injury” during ex vivo storage, and “ischemia reperfusion injury” if they contain antioxidants.8 I had come to the conclusion that the potential contribution to graft survival of holding solutions was relatively small compared to graft trauma and ischemia. If the potential benefit was in the range of a 5-10% increase in average graft survival, it would take a well–done, controlled clinical study of at least 50 patients to demonstrate this, something I could not do in my practice.
However, at least when it comes to holding solutions, we have a proxy way of testing their effectiveness. By extending the storage time, we can magnify the difference between various storage solutions and thereby increase the validity of any differences we observe. The assumption here is that if grafts held in storage solution A has drastically superior survival compared to grafts held in storage solution B after 48 hours in storage, then storage solution A probably has some unspecified benefit during the 2-8 hour storage times of a typical hair transplant.
So I tested my favorite holding solution, HypoThermosol FRS, both with and without the addition of the liposomal ATP during an extended storage study. The patient was a 70-year-old man whom I had been taking care of for many years for skin cancer. I had excised a skin cancer on his left temple and had him complete a course of radiation therapy to ensure eradication. This left a large area of complete alopecia in the area. We first excised the donor strip on day one, and dissected the grafts under the microscope per our usual protocol. We then divided the grafts into 3 groups: A) HypoThermosol +liposomal ATP, B) HypoThermosol without ATP, and C) PlasmaLyte A (normal saline pH 7.4), and stored them in these solutions for 5 days at 4°C. In addition, all of the areas were sprayed post-operatively with liposomal ATP, so the only difference was the storage solution.
The patient was followed periodically and final hair counts and photos were done at 18 months. Graft survival per area was: A) 72%, B) 44%, and C) 0%. HypoThermosol with liposomal ATP was the clear winner (Figure 3). While this study was only of a single patient, it is the longest survival study of hair ever reported (to my knowledge). And it does suggest that there would be some benefit even during shorter storage times (e.g., 2-6 hours) of a standard hair transplant.
Because I have such faith in HypoThermosol/ATP, I frequently use it for overnight graft storage when needed. For example, if we are doing a large FUE case and do not finish graft placement during a reasonable time, we simply store the grafts in the refrigerator overnight and finish placing the next day (Figure 4). We use tabletop electric chillers (available through Cole Instruments) to ensure grafts are at 4-8°C during the procedure, and, if necessary, store grafts in a standard refrigerator overnight so they can be placed the next day.
Why choose one holding solution over another? Why HypoThermosol as a graft holding solution, versus another solution such as culture media (e.g., Williams E, DMEM) or IV solution (normal saline, Lactated Ringer’s)? When tissue is stored at low temperatures, membrane pumps do not work properly, allowing sodium to rush inside the cell, followed by water. HypoThermosol, which was specifically designed for low temperature storage, prevents this from happening by holding water outside the cell.9 It also contains glutathione and synthetic vitamin E, which has been proven to prevent ischemia reperfusion injury.10 Finally, it is in widespread use for cell therapy applications throughout the world.
I have chosen HypoThermosol FRS because I believe it is the most rational choice. I accept that there are no large studies to prove which one is best for hair transplantation, but we can look at what evidence is available and make the best choice in our practice. If we are doing a very large case lasting over 12 hours, or on those rare occasions when we need to store the grafts overnight, I have complete confidence that HypoThermosol FRS is providing the best environment for my grafts.
ECM
Five years ago, I began experimenting with ACell MatriStem, a commercially available extracellular matrix (ECM) derived from porcine urinary bladder matrix (UBM). Reports continue to appear in the peer reviewed literature confirming the efficacy of UBM for a variety of purposes, such as in muscle regeneration, treating non-healing leg ulcers and as a dressing after skin flap failure.11-13
I reviewed my experience with ACell in a previous issue of the Forum,14 where I noted that ACell was useful for the following situations:
FUT strip healing: Does not change the appearance of the scar but promotes a softer, more natural feeling result that is easier to re-excise in future procedures (if needed).
FUE donor sites: Promotes regeneration if there are any transected follicles remaining in the site, prevents fibrosis, subsequent FUE sessions are easier.
Graft coating: Graft growth appears more robust, promotes angiogenesis around graft, and prevents recipient bed fibrosis.
My experience over the last several years has confirmed these observations. ACell is known to activate local stem cells, suggesting a role in helping damaged follicles regenerate. While none of us like to admit that despite our best efforts, some of our grafts are being damaged during placement, it is reassuring to know that grafts coated with ACell have a better chance of regenerating.
I would like to emphasize the “anti-fibrotic” action of ACell because I think it is one of the most important benefits of using this product in hair restoration. When taking a strip out in a patient who has had prior strip surgery, whether ACell was used in the previous surgery is abundantly obvious: the ACell scar is much easier to excise and feels more like virgin scalp, compared to the non-ACell scar, which feels like cutting through a rubber tire. Likewise when doing FUE on someone who has had ACell in their previous FUE, the skin is soft and more like virgin scalp, whereas the non-ACell patient’s skin is tougher, and dulls the punch quicker. I would imagine that transection rates are lower as well in patients who have had prior FUE + ACell.
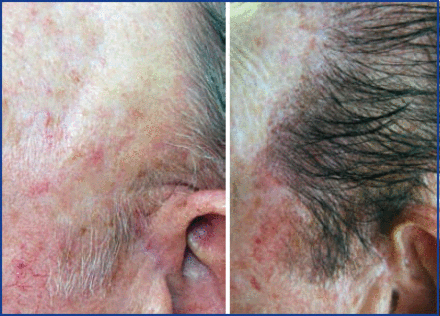
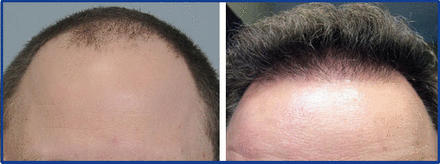
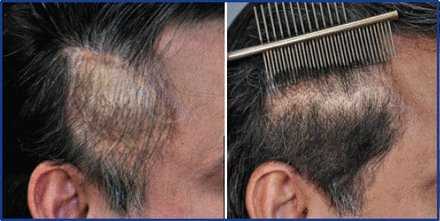
Using ACell-coated grafts helps protect and rejuvenate the recipient bed as well. I have been impressed with ability of ACell to reverse scarring and improve vascularity in scalps that have “old work” (plugs, mini-grafts, etc.) (Figure 5). I believe there is better protection for surrounding pre-existing hair (Figure 6) and that increase vascularity will lead to better growth in future procedures. Dr. David Seager pioneered the “one pass” density result because he believed micro-fibrosis would hinder growth when transplanting into the same area a second time. I believe this is less of a concern when ACell is used.
Following is how we use ACell in our office:
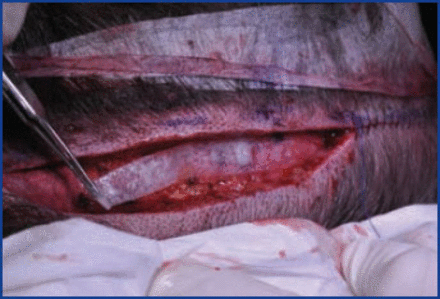
FUT donor: We take a 3×7cm sheet and cut it length wise into strips, and place these deep in the wound bed and suture the skin over it (Figure 7).
FUE donor: We inject PRP+ ACell into the donor area after harvesting, as well as placing some topically, and cover it with kitchen cellophane overnight.
Grafts: We create a concentrated suspension by adding a small amount of saline to the powder; a half a drop of this suspension is added to a pile of grafts on the placer’s finger prior to placing (Figure 8).
Treatment for miniaturizing hair: PRP+ ACell. We add 50-75mg of ACell to our platelet rich plasma (PRP) prior to injection. If done at the same time as the transplant, we inject the PRP/ACell after the sites are made and before the grafts are placed.
Regarding this latter application, we have been doing more and more of these procedures in the last couple years. Several reports have appeared in the peer reviewed literature reporting improvements in hair following PRP,15-16 which adds to Greco’s original clinical observations.17 My clinical impression is that we can usually achieve mild to moderate thickening beginning at 6 months and maturing at 12 months (similar to a transplant). While the results can vary, it seems that the greater the percentage of miniaturizing hairs, the greater the chance for improvement. When patients ask me how long the benefits last, I answer that it depends on two important factors: 1) their underlying genetics (e.g., balding fast vs. balding slow), and 2) what hair treatments they are on (e.g., results last longer if patient is on finasteride and minoxidil). There is much we do not know about this procedure but the combined experience of those of us doing PRP as a thickening treatment for AGA suggests it is useful and here to stay.
Conclusion
We currently use liposomal ATP, ACell, and HypoThermosol on virtually every case. We only use PRP/ACell when there is a significant amount of miniaturized native hair. I’m convinced that not only does each product contribute significantly to the final result, but that they are synergistic with each other as well. For example, the growth factors in ACell signal specific cellular actions that require ATP, hence the synergy with liposomal ATP. Over the past 10 years, I have gone through periods where I have used none of these, all of these, or varying combinations; my results are best when I use all three.
Some will question whether all of this is really necessary. I can merely state that these bio-enhancements have helped me improve my results. It is up to each individual surgeon to identify possible areas for improvement in their own results and to make a plan to address these. I’m reminded of the debates in the mid-1990s about whether microscopic dissection was really necessary. Many of us thought this was unnecessary at the time, but individual and collective experience over the years confirmed the superiority of the follicular unit approach. Time will tell whether these bio-enhancements are accepted in the same way. What happens will be determined by our shared clinical experience.
- Copyright © 2014 by The International Society of Hair Restoration Surgery